Pharmaceutical & Life Sciences
Pharmaceutical and life sciences companies face rigorous scrutiny from regulators, patients, and global stakeholders. SAI360’s pharmaceutical and life sciences software helps you manage GxP risk, monitor global compliance obligations, and embed ethical behavior—without slowing clinical innovation or market delivery.

Trusted by leading Pharma & Life Sciences companies
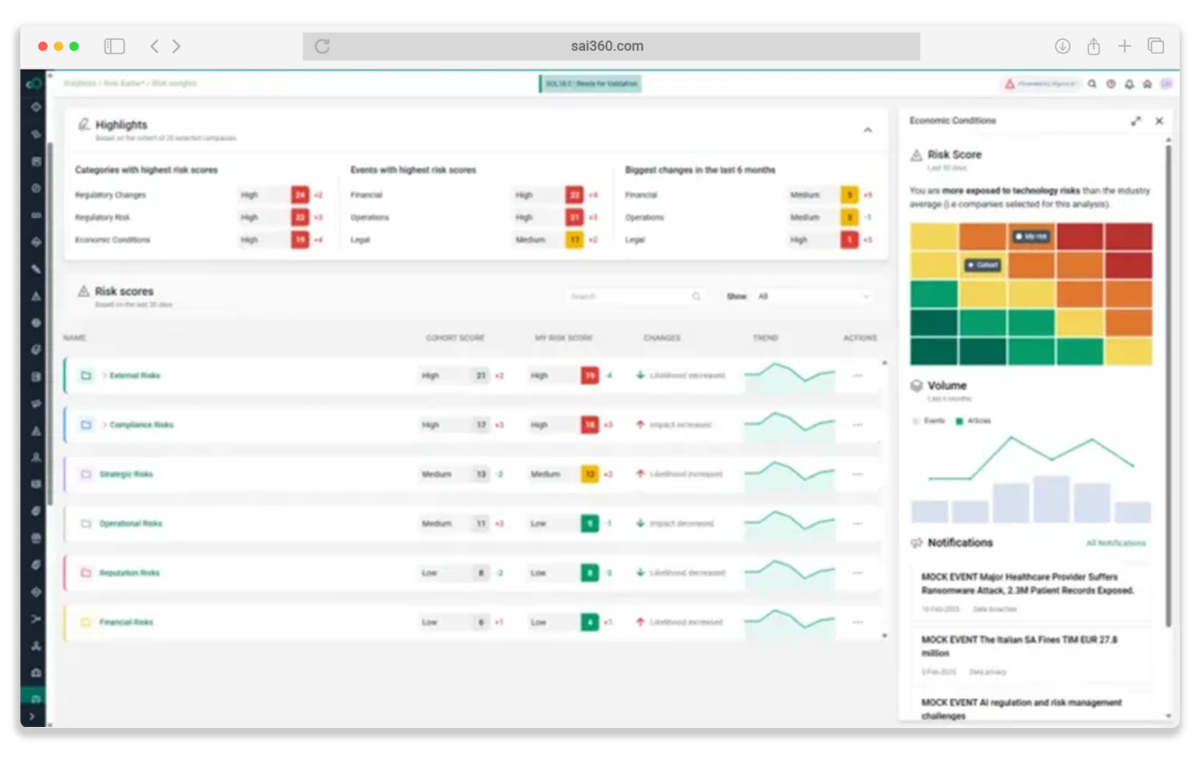
Connected Risk Visibility
Unify quality, regulatory compliance, and operational risk management from lab to launch with our pharmaceutical and life sciences software.
- Map risk across R&D, manufacturing, and commercialization in one system
- Monitor cyber, IP, and supply chain threats before they disrupt operations
- Automate global compliance tracking for HCP interactions, gifts, and hospitality
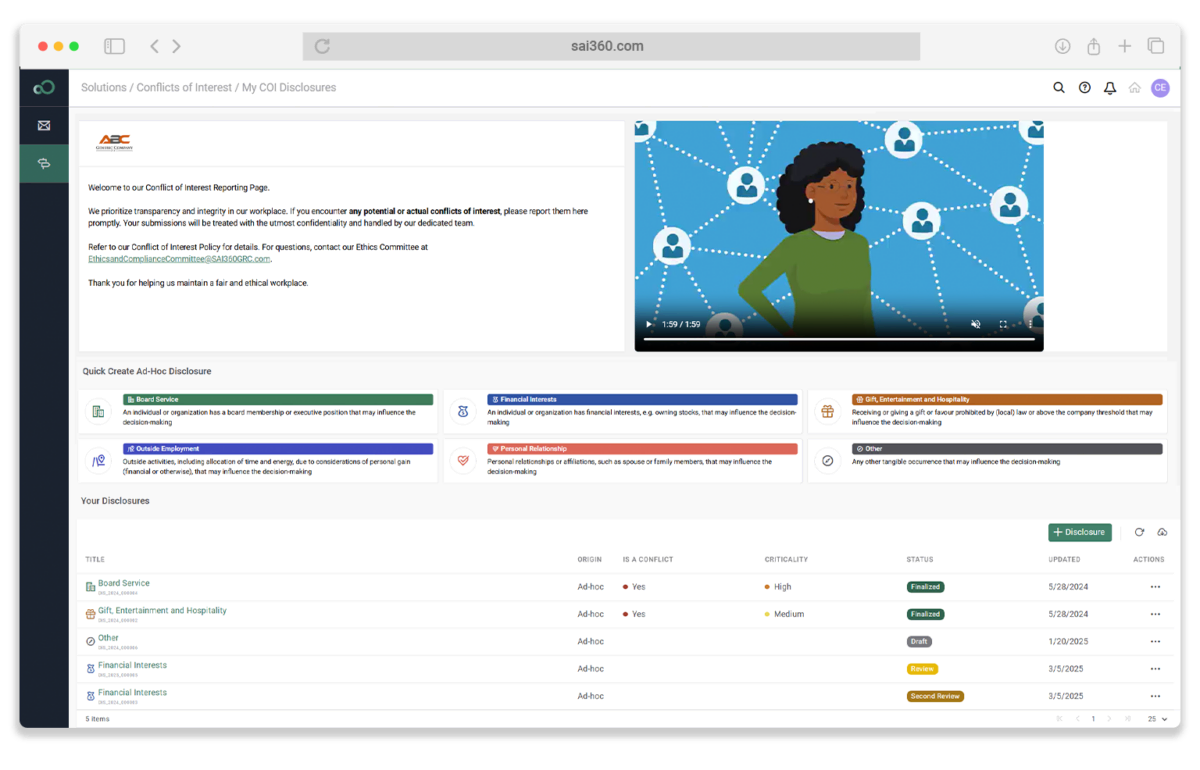
Compliance at Scale
Enforce controls, streamline disclosures, and elevate compliance culture across global teams.
- Centralize GxP-ready controls with built-in testing, evidence, and reporting
- Deliver role-specific training on data integrity, trials, and marketing standards
- Manage COI disclosures and attestations through embedded, policy-driven workflows
“At the end of the day, you want software that will simplify the complexities, keep you on top of changing regulations, protect privacy, and streamline processes–delivered by a team that understands your market and always has your back, which is why we work with SAI360.”
– Ryan Sloneker, Prime Therapeutics
How We Help Pharma & Life Sciences Organizations
Ready to strengthen compliance and protect your pipeline?